Consumer Product Safety Improvement Act.
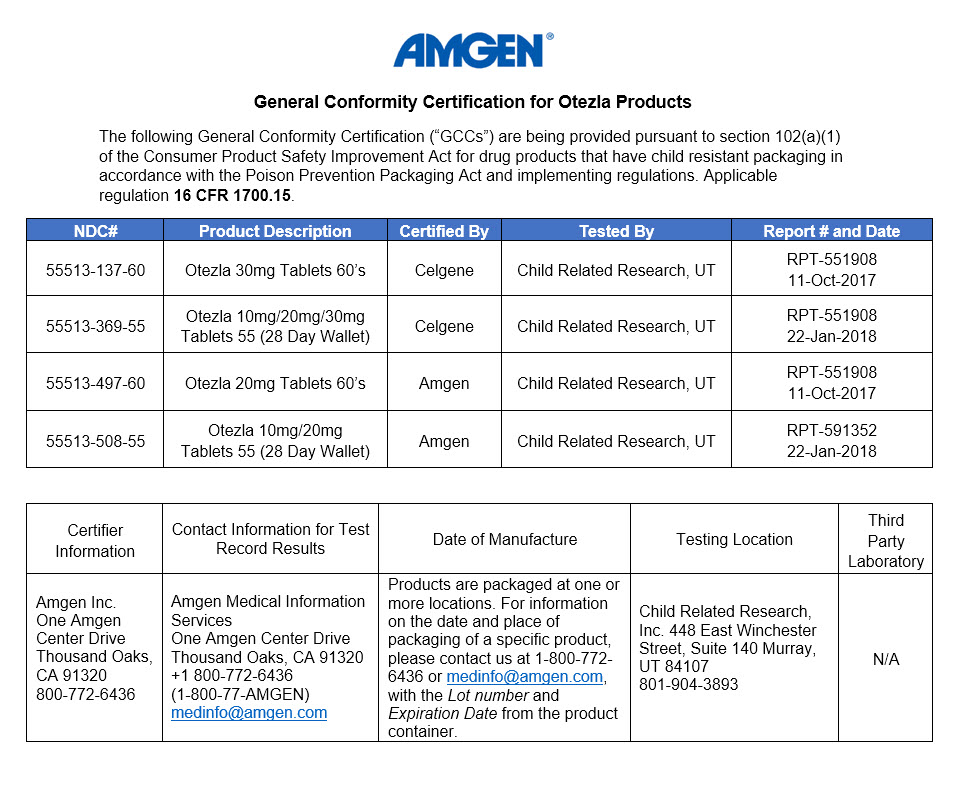
The Consumer Product Safety Improvement Act of 2008 (CPSIA) requires manufacturers and importers of certain consumer products (including certain drug products) manufactured on or after November 12, 2008, to certify that their products comply with all applicable rules, bans, standards, or regulations enforced by the Consumer Product Safety Commission (CPSC). Under the CPSIA, manufacturers and importers must make available to customers a certificate specifying that the product complies with the applicable CPSC rule, ban, standard, or regulation. Such certification must be based on a test of each product or upon a reasonable testing program.